Let's get started
We'll make sure you're getting the best out of your filtration system
SupaGard
Large European Manufacturer of Medicines & Dermo Cosmetics
Clarification of Cutaneous Medicine
Global
Validation Support
Pharma / Healthcare
It’s not just the filtration of parenteral drugs that have strict validation requirements in terms of product safety. Non-prescriptive topical treatments such as those for alopecia and male pattern baldness also require appropriate levels of safety.
A major manufacturer of an alopecia treatment based on the API Minoxidil is using the SupaGard filter to clarify the final product. While the quality of the SupaGard filter is supported by a comprehensive validation guide and is manufactured from materials conforming to FDA CFR21 and USP Class VI Plastics, a recent audit by Medicines Regulatory Authorities highlighted the need for additional testing.
They required documentary evidence that the filter was not altering the quality or safety of the final product based on their specific process parameters.
Often the exact extractable data required and protocols to be used are not well defined by the regulatory agency and the customer needs support to generate appropriate documentation.
We work with an experienced network of laboratory service companies specialising in cGMP filter validation and together with the customer, generate protocols and final validation reports that can be submitted directly to the regulator. This approach gives us access to a wider knowledge base for protocol development in tandem with fast turnaround times.

The objective was to prove that no extraneous contamination was extracted from the filter when processing the product. To do this, a model solvent was developed based on the product formulation to accurately mimic the activity of the solution containing the API. To simulate worst case conditions the extraction took place at a temperature 5oC above that of the production process.
The model solvent for the 5% API concentration is shown below:
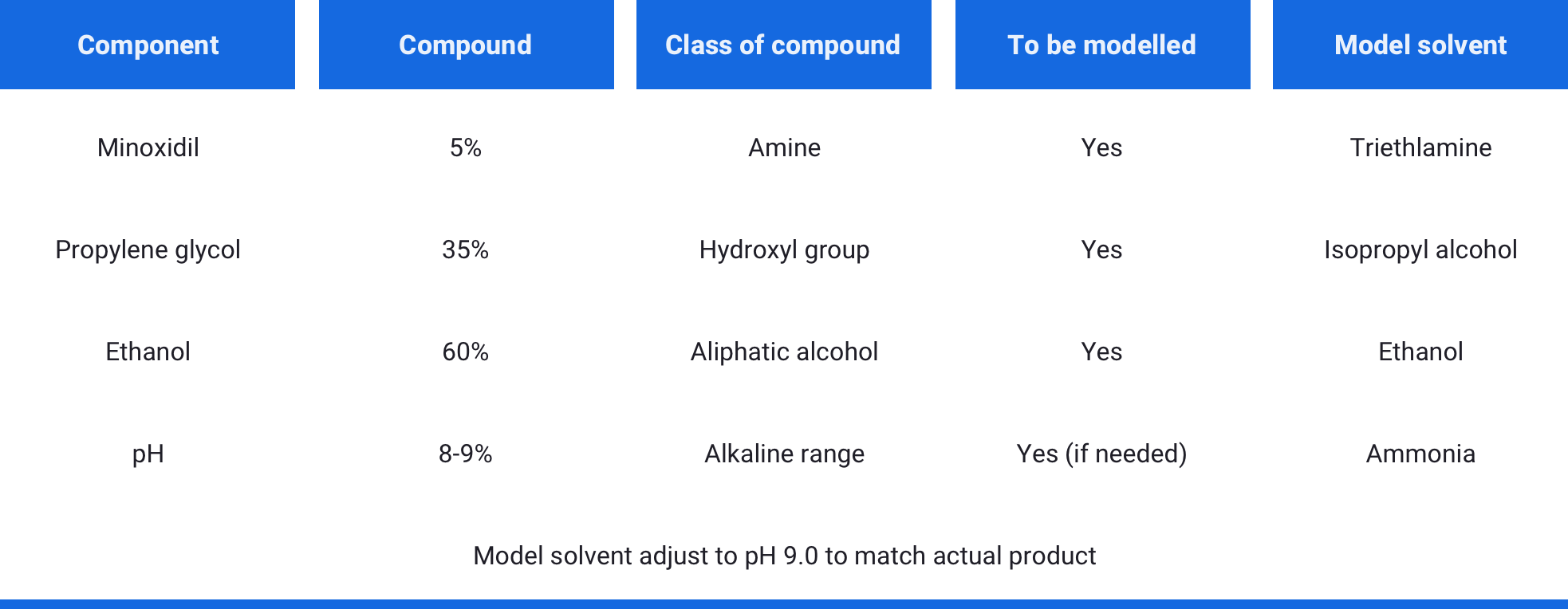
Tests were completed in triplicate and following exposure to the model solvent for a time matched to the filtration process in production. The non-volatile residue (NVR) were then determined along with the chemical identification of the extracted material via FTIR.
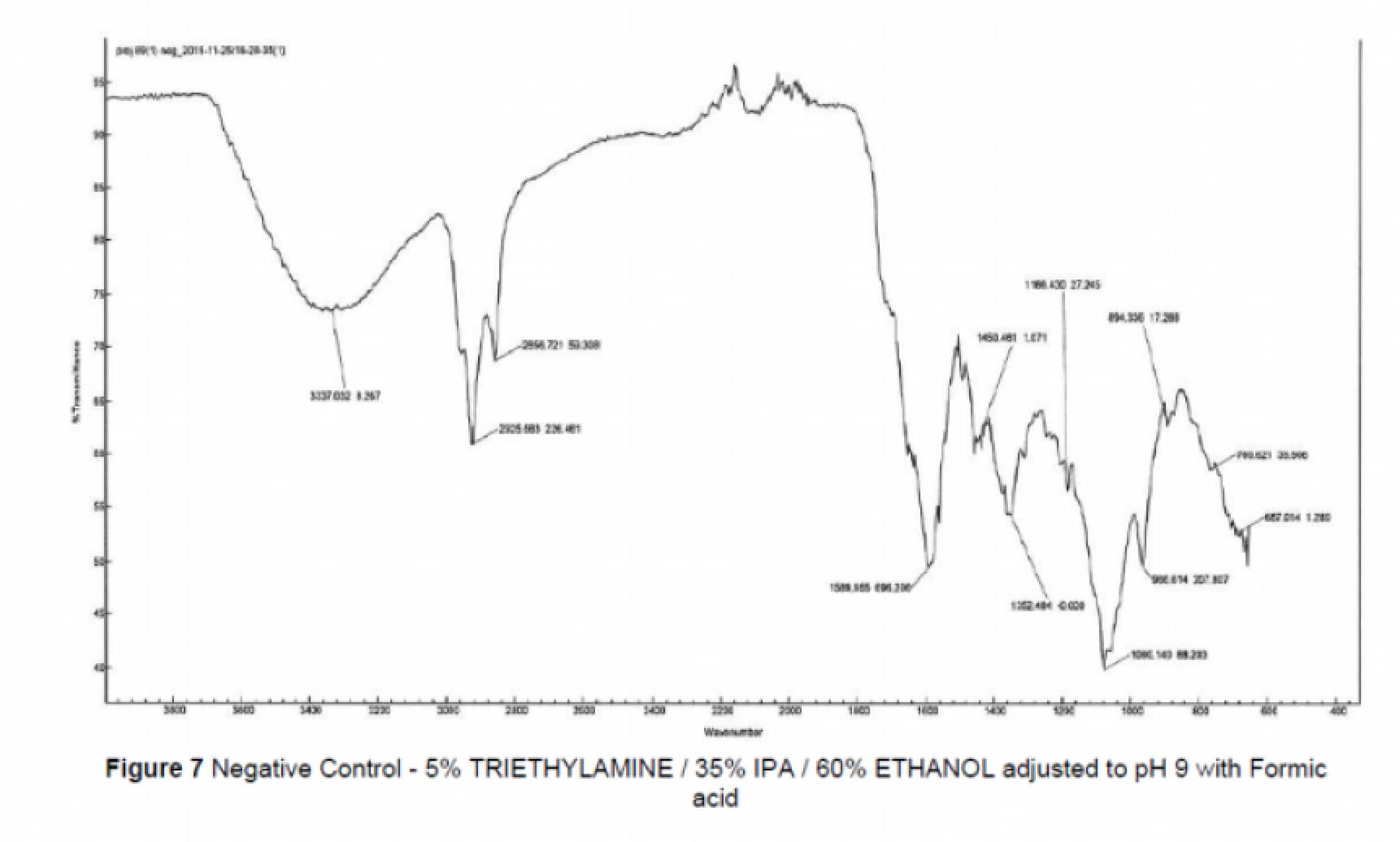
It has been demonstrated that extracted compounds from the SupaGard, under simulated worst-case process conditions, emanate only from the materials of construction of the test filter (polypropylene) and at extremely low levels (10µg/l of product filtered). The filter therefore does not affect the quality of the specific product.
The fact that no extraneous contamination was detectable on the filter demonstrates the overall cleanliness levels of the filters supplied to the customer and is testament to the quality procedures and clean room manufacturing facilities at Amazon Filters.
The final report was readily accepted by the regulatory auditor as proof of the filter being applicable for the process.

Amazon Filters is able to support filter users in generating the full range of product and process specific Validation Reports required by Medicines Regulatory Authorities. In addition to the Extractables Testing detailed in this case study Amazon Filters is also able to partner with manufacturers to generate Validation Reports on Bacterial Removal Efficiency, Compatibility Testing and Active Ingredient Adsorption assessment.
For more information, please contact us using the form below, or give us a call on +44 (0) 1276 670600.
We'll make sure you're getting the best out of your filtration system